Chimeric antigen receptor T cell (CART) immunotherapy has been accumulating extraordinary breakthroughs in fighting against hematological malignancies. Particularly, universal, “off-the-shelf”, CARTs (uCARTs) are emerging as an attractive alternative to patient-derived CARTs, offering cost-effectiveness, immediate availability for treatment, and independence from patients' lymphopenic conditions. However, uCARTs share the same safety concerns with autologous CARTs while the efficacy and persistence of uCARTs vary by each product and remain uncertain. Further, T cells sourced from healthy donors for uCARTs may manifest individual-specific immune characteristics upon interaction with cancer cells and the patient's immune system. These characteristics often remain latent when assessed in vitro and complicate the prediction of uCART therapy's toxicity and efficacy on an individual basis.
We previously demonstrated that the PBMC-humanized mouse model can capture efficacy and toxicity of autologous and allogeneic CD19 CARTs. We showed that the CART-induced toxicity varied by the combination of PBMC donors and CART cell donors. Here, we extend the use of PBMC-humanized mice to assess the potential toxicity, efficacy, and persistence of uCARTs.
Female NSG™-DKO mice (n = 19-20 mice per donor) were irradiated and reconstituted with human PBMCs sourced from four selected donors (Donor A, B, C, and D) out of 58 available in our PBMC bank. The selection criteria were based on their pre-characterized in vivo human cytokine induction profiles, specifically IFN-gamma response levels to OKT3 treatment (Donor A: 268 pg/ml; B: 1936 pg/ml; C: 4862 pg/ml; D: 13538 pg/ml).
PBMC-humanized mice were grouped into three different CD19 CART treatments; a) Allogeneic CARTs were manufactured from a healthy donor's T cells containing CD28 costimulatory domain (19-28z) and no genetic modification was made for “off-the-shelf” availability; b) uCART 1 cells (19-28z) were knocked out of TCRab via CRISPR-cas9 (19-28z); c) uCART 2 cells were knocked out of CD3 and TCRab using TALENs and magnetically depleted for remaining CD3/TCRab, and contain 4-1BB costimulatory domain.
Body weight change and clinical scores were measured throughout the experiments. We measured the levels of human CD45, human CD19, and CART expansion from blood collected 8 days post-treatment using flow cytometry. We measured 54 human cytokine levels from serum collected 48 hours post-treatment using a multiplex assay.
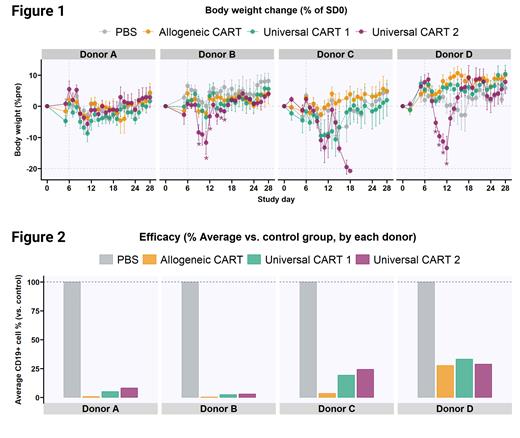
CART-induced toxicity, measured by body weight loss, dramatically varied by PBMC donors and the treatments (Figure 1). uCART 2 induced significant body weight loss in Donor B and D reaching the lowest 6 days post-treatment. Notably, all mice humanized with Donor C and treated with uCART 2 reached a humane endpoint within 12 days post-treatment. We only observed noticeable body weight loss from uCART 1 in Donor C. Allogeneic CART did not induce body weight loss across the four donors.
Efficacy, measured by the % of elimination of human CD19 cells in peripheral blood compared to the PBS-treated group, also varied by PBMC donor and the treatments (Figure 2). Overall, Allogeneic CART showed the best elimination of CD19 cells. Mice humanized with Donor D responded with the poorest efficacy of the three CART treatments. CART expansion was observed only in uCART 2 treated mice that reached a humane endpoint within 6 days post-treatment. CART persistence measured 16 days post-treatment was not observed across three CARTs and this corresponds to the clinical data of uCARTs.
Principal component analysis of human cytokine data revealed that uCART 2 induced the most distinct cytokine responses compared to the other CARTs, driven by IFN-gamma, IL-10, IL-5, IL-3, and GM-CSF. In Donor-A humanized mice, the same set of human cytokines (IFN-gamma, IL-10, IL-5, IL-3, GM-CSF, IL-13) was induced in response to the three CARTs, while the degree of induction varied by treatment. In contrast, Donor D-humanized mice showed vastly different responses to each treatment; uCART 2 induced significant levels of IL-6, TNF-beta, MIG/CXCL9, MDC, MCP-3, IL-13, and IL-2.
In sum, we show that PBMC-humanized mice capture individual variations in the toxicity and efficacy of uCARTs. This platform can be used to preclinically assess efficacy and persistence of uCARTs as well as used to predict toxicity from the interaction between the host and healthy T cell donor of uCARTs.
Disclosures
Jackson:Artisan Bio: Current Employment. Ott:Sana Biotechnology: Current Employment. Gardner:Bristol-Myers Squibb: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal